Functional Cartilage Tissue Engineering with TGF-beta
-
Promising osteoarthritis treatment strategy whereby cell-seeded constructs are fabricated to form neo-hyaline-cartilage to repair patient tissue defect.
-
Transforming growth factor beta (TGF-beta) is an anabolic hormone used to promote rapid cartilage ECM biosynthesis.

-
TGF-beta is conventionally administered at highly supraphysiologic doses in an effort to accelerate development of neocartilage to achieve load-supporting functional tissue properties.
-
However, supraphysiologic TGF-beta growth rates may come at a cost, leading to pathogenic features in the form of cell hyperplasia or fibrosis.
Supraphysiologic TGF-beta Induced Neocartilage Pathogenesis?



-
Supraphysiologic TGF-beta doses can give rise to undesirable neocartilage growth outcomes:
-
Tissue growth suppression
-
Hyperplasia-induced cell clusters
-
Increased fibrocartilage-specific type-I collagen
-
-
Physiologic TGF-beta doses give rise improved tissue quality:
-
Native material properties
-
Improved cell morphology
-
Exclusive hyaline cartilage type-II collagen
-
Bio-inspiration for Controlled TGF-beta Delivery
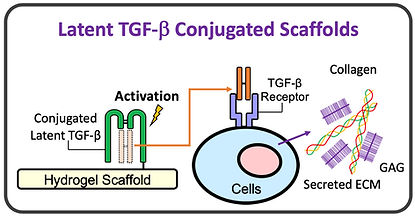
-
Bio-inspired scaffolds capitalize on native regulatory mechanism, whereby TGF-beta is delivery to chondrocytes in inactive, latent complex (LTGF-beta).
-
LTGF-beta is slowly activated by chondrocytes, giving rise to tightly regulated physiologic activity, promoting growth while avoiding pathology induced by TGF-beta excesses.
Enhanced Cartilage Regeneration via LTGF-beta Scaffolds

-
LTGF-beta conjugated scaffolds give rise to improved cartilage regeneration outcomes relative to that achieved via media supplementation at physiologic (0.3ng/mL) and supraphysiologic (10ng/mL) doses, including engineered cartilage with:
-
1) Native-matched mechanical properties (EY > 600kPa)
-
2) Improved uniformity of composition and mechanical properties, needed to support physiologic loading
-
3) Improved cell morphology evidenced by reduction in pathologic cell clusters
-
-
Growth stimulation via LTGF-beta scaffolds represents a paradigm shift in musculoskeletal regenerative medicine, allowing for engineered tissues with improved composition, structure, and mechanical function
Related Publications
Peer Reviewed Journal Articles
-
Albro MB, Nims RJ, Durney KM, Cigan AD, Shim JJ, Vunjak-Novakovic G, Hung CT, Ateshian GA. (2016) Heterogeneous growth of engineered cartilage results from gradients of media-supplemented active TGF-beta and is ameliorated through the alternative supplementation of latent TGF-beta. Biomaterials, 77: 173-185.
-
Albro MB, Li R, Banerjee RE, Hung CT, Ateshian GA. (2010) Validation of theoretical framework explaining active solute uptake in dynamically loaded porous media. Journal of Biomechanics, 43(12): 2267-73.
Conference Abstracts
-
Wang T, Martin A, Dai Z, Liu Y, Murphy-Ullrich JE, Grinstaff MW, Albro MB. (2022) A bio-inspired latent TGF-beta conjugated hyaluronan scaffold for cartilage tissue engineering. Annual Meeting of the Orthopaedic Research Society, abstract no. 217.
-
Lyu Y, Wang T, Liu Y, Albro MB. (2022) Culture medium replenishment is not required to generate functional engineered cartilage in vitro. Annual Meeting of the Orthopaedic Research Society, abstract no. 1502.
-
Wang T, Martin A, Dai Z, Liu Y, Murphy-Ullrich JE, Grinstaff MW, Albro MB. (2021) Latent TGF-beta conjugated scaffold improve functional properties and cell morphology in engineered cartilage. Next Generation of Tissue Engineers Trainee Symposium.
-
Zhang J, Wang T, Jensen M, Grinstaff MW, Snyder BD, Bergholt MS, Albro MB. (2021) Raman needle arthroscopy towards in vivo monitoring of engineered cartilage growth. Summer Biomechanics, Bioengineering, and Biotransport Conference (SB3C), abstract no. 562.
-
Wang T, Dai Z, Dogru S, Kim SY, Albro MB. (2021) Localized delivery of physiologic TGF-beta doses improves cell morphology in functional engineered cartilage. Annual Meeting of the Orthopaedic Research Society, abstract no. 713.
-
Wang T, Berigei S, Wang Y, Grinstaff MW, Murphy-Ullrich JE, Albro MB. (2019) A latent TGF-beta scaffold improves engineered articular cartilage quality. 65th Annual Meeting of the Orthopaedic Research Society.
-
Wang T, Sharifikia D, Baeyens S, Albro MB. (2018) Localization of delivery of moderated, near-physiologic levels of active TGF-beta can produce engineered cartilage of improved tissue quality. 2018 World Congress of Biomechanics.
-
Albro MB, Spicer CD, Ballinger M, Medina A, Littmann E, Stevens MM. (2016) Strategies for growth factor conjugation to acrylate-modified agarose scaffolds for cartilage tissue engineering. 62nd Annual Meeting of the Orthopaedic Research Society.
-
Albro MB, Nims RJ, Durney KM, Cigan AD, Shim JJ, Vunjak-Novakovic G, Hung CT, Ateshian GA. (2015) Heterogeneous growth of engineered cartilage results from gradients of media supplemented active TGF-beta and is ameliorated through the alternative supplementation of latent TGF-beta. 61st Annual Meeting of the Orthopaedic Research Society.
-
Albro MB, Cigan AD, Nims RJ, Chen YBC, Hung CT, Ateshian GA. (2012) Synthesis and incorporation of latent TGF-binto the extracellular matrix of engineered cartilage constructs. 58th Annual Meeting of the Orthopaedic Research Society.